You’ve likely seen anodized aluminum products in your daily life. Anodizing is an easy way to add corrosion protection and a splash of color to aluminum. You’ll find this finish on sports equipment, automotive parts, handheld devices, and a wide range of other products.
If you’re wondering whether anodizing is the best way to finish your aluminum products or would like to know more about the process and its benefits, this article is for you.
Here we answer some of the most commonly asked questions about anodizing aluminum to help you decide if this process is the right choice for your products.
Table of Contents
1) What is Anodized Aluminum?
Exposing metals to certain environments such as air and humidity creates a metal-oxide layer on the exposed surface. In most cases, the oxide layer is passive, which means it no longer reacts with the environment the way the pure metal does. The rust that forms on iron when it’s left unprotected is one example of this.
Like iron and other metals, aluminum naturally forms a layer of oxide when in contact with air or moisture. Unlike iron, however, the layer of oxide formed is not flaky or overly porous, and it serves as a shield preventing any further oxidation of the aluminum.
In other words, aluminum creates its own thin barrier against corrosion by slightly corroding itself. This is similar to how human skin tans to protect itself from further damage caused by sun rays or other ultraviolet light.
When you anodize aluminum, it goes through a process that thickens this naturally-occurring protective layer of oxide. Anodizing enhances the properties offered by the oxide layer, and these properties are addressed one-by-one in the following sections.
2) How Does the Anodizing Process Work?

Contrary to intuition, the process of anodizing doesn’t require technicians to apply a product to the aluminum surface. As explained before, this process takes advantage of a reaction that occurs naturally on aluminum when in contact with certain elements and takes it up a notch.
Once the aluminum has gone through a forming process — extrusion, for example — the resulting aluminum part is submerged in an electrolytic bath. While immersed in the liquid, a high-amperage, low-voltage electrical current is applied to the bath. The oxidation chemical reaction occurs as the current flows through the aluminum, forming an oxide layer thicker than the naturally occurring one.
3) Does Anodizing Increase Resistance to Wear?
Yes, anodizing does improve wear resistance. The oxide layer that forms in the aluminum surface has ceramic properties, one of which is a heightened resistance to wear compared to the aluminum on its own. Anodized aluminum is better than untreated aluminum at resisting nicks and scratches, providing a more durable finish.
4) Does Anodizing Increase Corrosion Resistance?
Yes, it does. Like the naturally occurring oxide passive film, the thick layer of oxide obtained by the anodizing process also improves corrosion resistance. The passive film is largely chemically inert and does not break down or corrode in the environment.
As a result, the anodized layer protects the underlying aluminum from corrosion. Anodized aluminum has a thicker passive layer than naturally passivated aluminum, meaning it is more resistant to degradation and subsequent corrosion.
5) How Do You Dye Anodized Aluminum Parts?
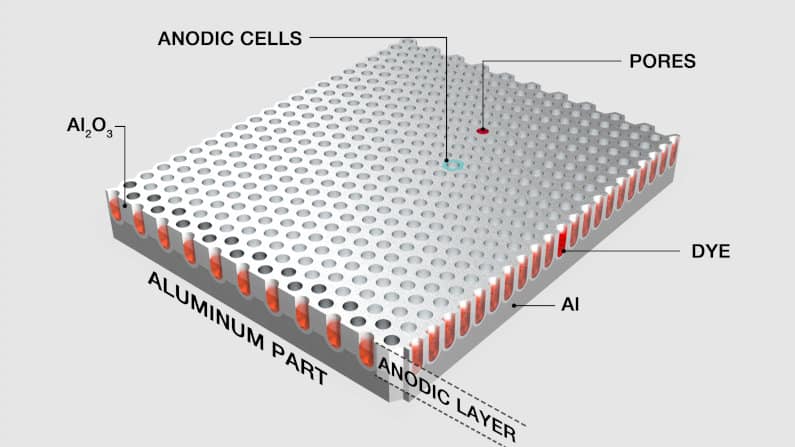
Another benefit of anodizing aluminum is that the metal’s surface becomes porous. Micropores make the anodized aluminum perfect for dye application — they act as wells where the dye can collect.
Once the dyes are applied, the pores can be sealed, resulting in the color being embedded in the oxide layer. Because the dye is now part of this passive layer, it will not fade or peel away, providing a durable, long-lasting, and elegant finish.
6) Does Anodizing Improve the Adhesion of Primers and Glues?
Yes, for the same reason that anodized aluminum is excellent for dyeing. The porous surface of anodized aluminum means the glue or primer has an increased contact surface and better spots to “grab on.” The pores allow the glue or primer to partially embed into the surface, resulting in an even layer of glue or primer with great adhesion that is less likely to spall off.
7) Does Anodizing Improve Heat Dissipation?
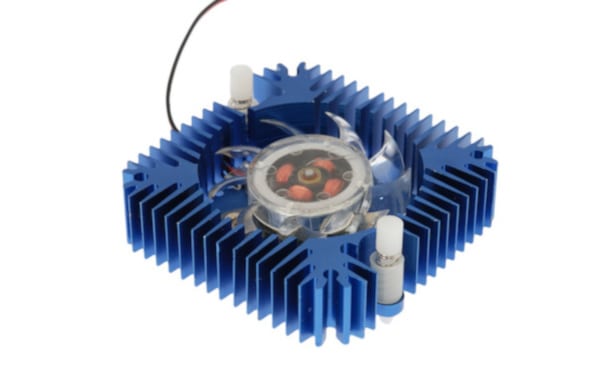
Yes, it does. If an item is hotter than its surroundings, it will start to cool off. The greater the surface area of a hot item, the quicker it will dissipate its heat. Anodized aluminum has a higher surface area than unfinished aluminum and, therefore, it is more effective at releasing its heat.
This improved thermal conductivity, or heat dissipation, results from improved convective heat transfer and enhanced emissivity. Convective heat transfer, which is heat transfer between a surface and the surrounding air, is primarily impacted by design and somewhat by anodizing.
Radiative heat transfer, also called emissivity, occurs between two surfaces and is dramatically improved by anodizing. This property makes anodized aluminum perfect for small heat sinks, as described by our article about anodized aluminum heatsinks here.
8) Does Anodizing Affect the Strength of the Aluminum?
No, it does not. The aluminum product’s strength will not be affected, either positively or negatively, by anodizing it. The process of anodizing affects only a very small layer on the aluminum surface, on the scale of micrometers.
Since the bulk of the aluminum part remains unchanged, the product retains the properties obtained by the forming and treatment applied before anodizing it.
9) Does Anodized Aluminum Have High Electrical Conductivity?
No, it does not. Aluminum is highly conductive by itself; however, the oxide layer has physical and chemical properties resembling a ceramic. One of the principal characteristics of ceramics is electrical insulation.
An anodized aluminum piece is still capable of limited electrical conductivity through contact, as the oxide layer is very thin, but the conductivity is significantly lower relative to untreated aluminum. While there are workarounds, you may want to consider a different finishing treatment for your aluminum products if electrical conductivity is an essential aspect of the item’s design.
10) What’s the Difference Between Type II and Type III Anodizing?
The anodizing process requires the proper selection of many variables: bath type, temperature, voltage, amperage, etc. Because of this, certain specifications are necessary to ensure the desired outcome is achieved consistently.
The most common way to specify different types of anodizing is by the military specification for anodic coatings for aluminum and aluminum alloys MIL-A-8625. In broad terms, this document separates the types of anodizing into Type I, II, and III.
Type I anodizing, the oldest known method, is performed in a bath of chromic acid. Type II and III are done in sulphuric acid.
The difference between Type II and III is the thickness of the resulting oxide layer. Type II anodizing, being the most commonly applied, has a layer with a thickness ranging between 1.8 to 25 micrometers. Type III anodizing, also known as hard anodizing and used where increased wear and corrosion resistance is desired, consists of oxide layers thicker than 25 micrometers.
Should You Anodize Your Aluminum Extrusions?

Choosing whether or not to anodize your aluminum extrusions depends on the intended applications for the products. As this article has highlighted, there are both benefits and drawbacks to anodizing.
Suppose your main concerns are corrosion resistance and a very nice, metallic look, or you’re looking to improve emissivity or adherence of primers or glue. In that case, anodizing could be ideal for finishing your extruded products.
However, anodizing might not be the best option if electrical conductivity is essential or if further forming processes are to be performed on the piece. These might cause the oxide layer to crack. Anodizing also slightly increases the dimensions of the piece. Therefore it is not recommended if you are working with very tight size tolerances.
If you are looking for alternatives to anodizing, you might consider using powder coating as a way to finish your aluminum items, giving them a bright colored finish. If a matte finish works for your application, and you need more extended durability and protection against UV fading, you might consider PVDF coating your aluminum products.




